Let us help you "Get to Done"
We’ve been in your shoes. Let us help you find a better way.
The No Surprises Act (NSA) introduces new protections and a transparent framework for resolving out-of-network payment disputes, empowering health plans and providers to navigate these challenges via a multi-step process. The Independent Dispute Resolution (IDR) process, while vital for resolving out-of-network payment disputes under the No Surprises Act, comes with its own set of challenges:
Managed by the Departments of Health and Human Services, Labor, and the Treasury, this system supports fair billing practices while striving to foster collaboration between health plans and care providers.
To ensure a seamless experience, health plans and providers must adopt proactive strategies, such as streamlining communication workflows, standardizing templates, and implementing robust tracking systems to stay on top of deadlines and deliver timely responses.
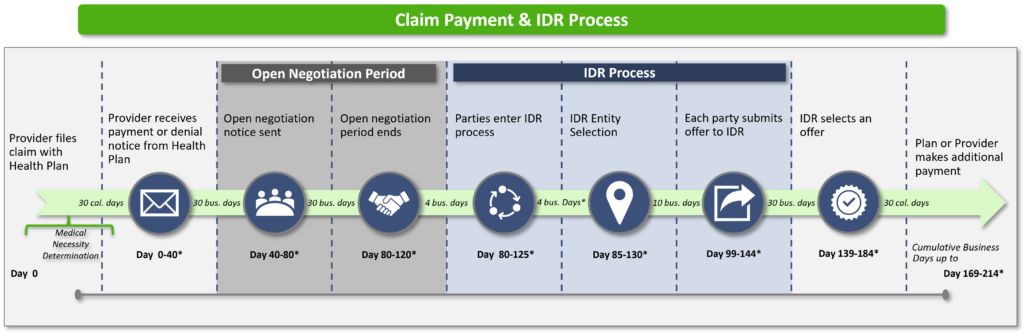
When managing an influx of claim disputes, non-standard email notifications, and required actions based on varied deadlines with the focus on compliance and revenue cycle goals, it is best to reassess and redesign your NSA/IDR process.
Reviewing a difficult process requires a structured approach to identify inefficiencies, pinpoint root causes, and develop actionable solutions.
Here are the key assessment steps:
Trexin can help you navigate and overcome the varied challenges in the IDR process with results focused on process efficiency, submission compliance, and financial success.
 Case Study
June 3, 2025
Case Study
June 3, 2025
Healthcare & Life Sciences | Optimized Operations | Products & Distribution | Strategy & Innovation
Trexin helped a large MedTech leader survey its suppliers to fully inventory the presence of “forever chemicals” in their products.
 Case Study
April 10, 2025
Case Study
April 10, 2025
Healthcare & Life Sciences | Optimized Operations | Technology
Trexin conducted a comprehensive risk assessment of a health insurance subsidiary’s cybersecurity controls and resilience strategies.
 News
March 27, 2025
News
March 27, 2025
Data & Artificial Intelligence (AI) | Digital Transformation | Strategy & Innovation | Technology
Trexin Consulting, a national management consulting and technology solutions firm specializing in digital strategy execution, announced today that Glenn Kapetansky has been elevated to Senior Member status with the Institute of Electrical and Electronics Engineers (IEEE).