A Strategy for Monitoring and Detecting Drug Diversion
Understanding the medication lifecycle.
To understand where and how drug diversion may occur, one must first understand the medication lifecycle. The medication lifecycle in an acute care setting starts by placing an order with the wholesaler to procure the medication and ends when it is administered to the patient. The timeline can be divided into two phases. The first phase consists of pharmacy driven processes. This includes ordering, receiving, storing, and dispensing medications. The second phase is any non-pharmacy processes involving medications, including nursing and anesthesia staff dispensing from automated dispensing cabinets, administering, wasting, and returning medications. This Trexin Insight Paper will describe how to monitor and detect diversion throughout each step of the medication lifecycle.
In recent years, drug diversion attempts have become increasingly complex. As healthcare institutions move to electronic and automated systems, users are looking for alternative ways to divert drugs undetected. Those looking to divert will exploit any step of the medication lifecycle in which they believe their actions will go unnoticed. To combat these efforts, it is critical that each step within the medication lifecycle is effectively and efficiently audited. When paired with the right policies and procedures, audits can detect most diversion attempts within the medication lifecycle.
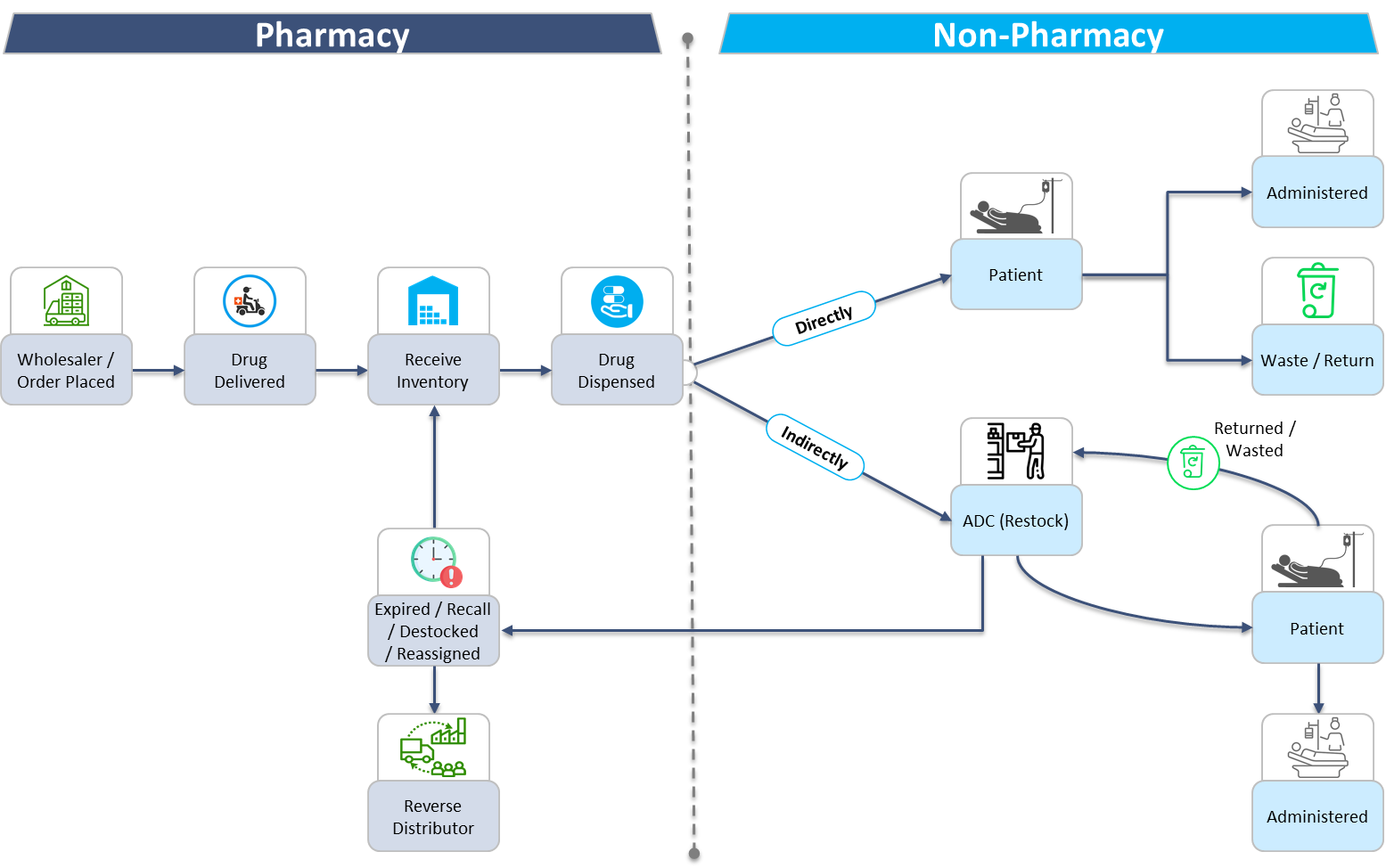
Phase 1: Pharmacy Processes
Each pharmacy driven process is linear, in that the expected outcome of each workflow does not change. Whether it is ordering medications, receiving inventory, or dispensing medications, the end result is always known. For example, if 100 tablets of a medication are ordered, the expectation is that 100 tablets are received into inventory. This makes auditing pharmacy processes quite simple. There are a few strategies to consider implementing within the pharmacy workflows and audits to further reduce the risk of drug diversion.
One strategy to consider is creating a separation of duties so no single person controls the medication throughout the entire lifecycle. For example, the person responsible for ordering the medications should be different from the person who receives the inventory. Similarly, the person auditing this step of the medication lifecycle should be entirely removed from ordering and receiving. Another consideration is to have a witness attest to certain tasks being accomplished correctly. For example, while receiving medications into the inventory count, two people should be involved in the process to ensure all medications are physically accounted for and properly placed into inventory. Even though these strategies help minimize the risk of drug diversion, each task must be regularly audited to verify diversion did not take place. Processes and audits need to definitively prove that all medications are accounted for at that specific moment in time. Whenever possible, time stamps and user information should be captured each time a medication is accessed or handled. Perpetual inventory systems and automated medication dispensing cabinets not only capture this information, but they also reduce the risk of diversion and make auditing much more efficient.
Phase 2: Non-Pharmacy Processes
The tremendous amount of variability introduced by clinical decisions makes nursing and anesthesia workflows much more complex to audit. The human element of patient care creates an environment in which the number of possible outcomes are endless. Therefore, workflows and policies should be strictly enforced to limit the number of expected outcomes. For example, medications should be dispensed by the person who is administering the drug, and the dispense should occur as close to the administration time as possible. In this example, it limits who is responsible for the drug and when the drug is expected to be dispensed and administered. Deviations from the policy standard could therefore be a cause for concern. Monitoring nursing and anesthesia transactions should focus on unexpected results and differences seen amongst peer groups due to these newly introduced variables. When considering the sheer number of transactions and the exponential number of outcomes, analytic diversion monitoring tools are considered the gold standard for nursing and anesthesia related activity. Analytic software programs help identify variances and anomalies in practice patterns that may suggest the user is attempting to divert the medications. Although these programs cannot guarantee the detected activity is diversion related, it allows the drug diversion program to narrow their focus. Much like a needle in a haystack, analytic software programs help make the haystack smaller so the needles can be identified more easily and efficiently. In essence, they reduce the amount of ‘expected’ transactions so the drug diversion team can hone in on the unexpected transactions.
By understanding the medication lifecycle, different auditing and monitoring tactics can be implemented at each phase to reduce the risk of drug diversion. When coupled with the right policies, workflows, and tools, monitoring the medication lifecycle for drug diversion becomes increasingly efficient. Although these general guidelines can be applied universally, they also need to be individualized based on the capabilities of the institution.
Contact a Trexin Advisor to find out how these principals may be adapted to help monitor and detect drug diversion at your institution.



